Atomic Mass Formula
The atomic mass of an element is equal to the weighted average of the isotopes for that element. Isotopes are atoms that have the same atomic number (# of protons) but a different number of neutrons. The number of protons determines the identity of the atom, and isotopes have identical atomic numbers, so the atoms are of the same element. The number of neutrons is not equal so the masses of the isotopes will not be the same. Atomic mass is measured in atomic mass units (amu), where one amu is roughly equivalent to the mass of a single proton or neutron.
The isotopes of an element do not occur in equal percentages in nature, so a weighted average must be taken in order to achieve the atomic mass of the element. Isotopes occurring in higher percentages will have a larger effect on the atomic mass, while isotopes occurring in lower percentages will have a smaller effect.
The atomic mass is found by multiplying the mass of the isotope by its relative abundance, then adding the individual masses together.
Atomic Mass Formula Questions:
1. Calculate the atomic mass of chlorine using the information provided in the following table.
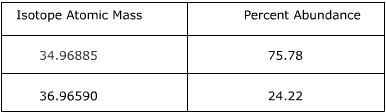
Answer:
To find the atomic mass of chlorine, the atomic mass of each isotope is multiplied by the relative abundance (the percent abundance in decimal form) and then the individual masses are added together.
34.96885 x 0.7578 = 26.50
36.96590 x 0.2422 = 8.95
26.50 + 8.95 = 35.45
The atomic mass of chlorine is 35.45 amu.
2. Calculate the atomic mass of Nickel using the information provided in the following table.
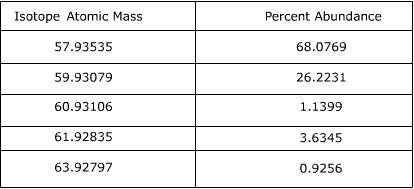
Answer:
To find the atomic mass of nickel, the atomic mass of each isotope is multiplied by the relative abundance (the percent abundance in decimal form) and then the individual masses are added together.
57.93535 x 0.680769 = 39.4406
59.93079 x 0.262231 = 15.7157
60.93106 x 0.011399 = 0.69455
61.92835 x 0.036345 = 2.2508
63.92797 x 0.009256 = 0.59172
39.4406 + 15.7157 + 0.69455 + 2.2508 + 0.59172 = 58.69337
The atomic mass of Nickel is equal to 58.69337 amu.
|
Related Links: Atomic Structure Subatomic Particles Quiz Atomic Theory I Quiz Atomic theory Timeline Fission vs. Fusion |
